28/1/03: Hydrogen bond and introduction to biological macromolecules
Hydrogen bond
An especially strong type of Van der Waals bond (with bond energy of
0.1 eV but the observed seperation between atoms with H-bond is smaller
than it would be with only VdW bond).
Neutral hydrogen has only one electron - so it is expected to form
a covalent bond with only one other atom. However, when bonded with the
most electronegative atoms (F, O, N), the electron of the H-atom is alomost
lost to the other atom. The bare proton of the H atomic nucleus can then
form a bond with another electronegative atom - the bond being largely
ionic in character. The atoms adjacent to the proton are so close that
more than two of them would get in each other's way - thus the H-bond connects
only 2 atoms.
H-bonds are much weaker than (say) covalent bonds - but when a large
number of H-bonds act in unison, they make a strong contributory effect.
The H-bond is an important part of the interaction between water molecules
and is responsible for many of the striking physical properties of water
and ice (including the hydrophobic effect and the unusually high melting
& boliling points of water).
Water molecules are exceptionally prone to form H-bonds because the
four pairs of electrons around the Oxygen atom in H2O are not
symmetrically distributed but are more likely to be found in certian regions
of high probability density - arranged along the vertices of a tetrahedron.
H atoms are at two of these vertices (which exhibit localised positive
charges while the other two vertices exhibit more diffuse negative charges).
Each H2O molecule forms H-bond with four other H2O
molecules. In the liquid state, these bonds between adjacent molecules
are being constantly broken and re-formed owing to thermal motion. Even
so, at any instant, the molecules are combined in definite clusters. In
the solid state, these clusters are large and stable and constitute ice
crystals. The tetrahedral arrangement of the four H-bonds that each H2O
molecule can participate in results in the characteristic hexagonal patterns
of an ice crystal.
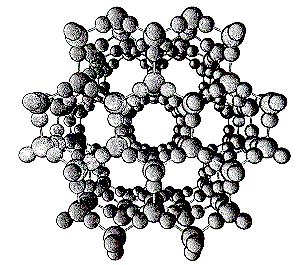 Structure
of Ice
Structure
of Ice
The hexagonal planar structure is responsible for the faceting planes
and six-fold rotation symmetry of snowflakes.
With only four nearest neighbours around each molecule (instead of
12 as in closest packing) ice crystals have extremely open structures.
This is responsible for the exceptional low density of ice [usually solids
are denser than the corresponding liquid - but ice is less dense than water,
which is why ice floats in water]. Because the molecular clusters are smaller
and less stable in the liquid state, water molecules are, on the average,
packed more closely together than are ice molecules. The density of water
increases from 0 oC to a maximum at 4 oC as
large clusters of water molecules are broken up into smaller ones that
occupy less space in the aggregate (only beyond 4oC, the normal
thermal expansion of a liquid manifests itself in a decreasing density
with increasing temperature).
Introduction to biological macromolecules
Hydrogen bond plays a very important role in biology. A few of the multiple
roles it plays are:
-
holding the two strands of DNA double helix together,
-
helping polypeptides (strings of amino acids) to form secondary structures
(e.g., alpha-helix, beta-sheet),
-
helping enzymes (bio-catalysts) bind to their substrate,
-
helping antibodies bind to their antigen,
-
helping transcription factors bind to DNA, etc etc.
Most importantly, Hydrogen bonding is responsible for the hydrophobic
effect.
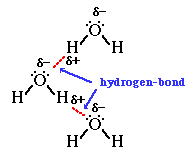 Hydrogen
bond between water molecules (the bond length is about 1.8 Angstrom) -
note the linearity imposed by the bond along the O-H-O axis (the angle
varies at most by 10 degrees from strict linearity, i.e., 180o).
Hydrogen
bond between water molecules (the bond length is about 1.8 Angstrom) -
note the linearity imposed by the bond along the O-H-O axis (the angle
varies at most by 10 degrees from strict linearity, i.e., 180o).
Water molecules orient so as to form H-bonds even
if the orientation restricts their mobility. So water molecules on the
surface (i.e., an air-water interface) will have fewer water molecules
with which to interact with than will molecules in the interior of the
solution. The smaller number of possible bonding partners will mean that
their possible orientations will be very limited in number - thereby lowering
the entropy (compared to molecules in the interior). To increase entropy,
water minimises its surface area. This is the reason for the high surface
tension of water.
Non-polar molecules (e.g., hexane) which interact
by VdW bonds and do not form H-bonds with water, also reduce the bonding
possibilities of adjacent water molecules. Therefore, as in the case of
air-water interface, the presence of non-polar molecules in water decreases
the entropy of the system. Exclusion of these nonpolar substances from
the aqueous solution will increase entropy. This entropy-driven effect
where nonpolar molecules are grouped together and excluded from the solution,
is known as hydrophobic effect - and non-polar molecules are known as hydrophobic
substances. Correspondingly, polar molecules which can form H-bond with
water are known as hydrophilic compounds. Note that nonpolar molecules
do not attract each other; they are pushed together because they are mutually
excluded from water.
For details on hydrophobicity, see the first few
pages of Thermodynamics
of Micelle Formation (Chapter 2, Fundamental
Principles of Membrane Biophysics) by David Njus.
Hydrophobic effect is the fundamental principle
for understanding biological membranes. Membranes give distinct identity
to a biological cell. A cell is a ``chemical machine" to transform
nutrients into building blocks for molecules with structural and functional
roles, like proteins, DNA, polysaccharides, etc. Transport across the membrane
is through active transport, which can occur even against a concentration
gradient (in contrast to passive transport). Membrane is composed of amphiphilic
molecules which are polar on one end and nonpolar on the other. By changing
their concentration in water, several kinds of structures can be formed.
In the cell, membranes are formed by phospholipids arranged in a
planar bilayer. Such a layer is impermeable to most substances - so to
allow the passage of molecules into and out of the cell the membrane also
has protein molecules embedded into it or attached to the surface (called
integral membrane proteins and peripherial membrane proteins
respectively). This is called the Fluid Mosaic model of membrane.
For further details on biological membranes,
see the lecture notes on Fundamental
Principles of Membrane Biophysics by David Njus.
The cell also needs a mechanism for storing information
about how to build molecules (e.g., the sequences of amino acids needed
to construct different proteins). This is stored in DNA.
 Structure
of Ice
Structure
of Ice
 Hydrogen
bond between water molecules (the bond length is about 1.8 Angstrom) -
note the linearity imposed by the bond along the O-H-O axis (the angle
varies at most by 10 degrees from strict linearity, i.e., 180o).
Hydrogen
bond between water molecules (the bond length is about 1.8 Angstrom) -
note the linearity imposed by the bond along the O-H-O axis (the angle
varies at most by 10 degrees from strict linearity, i.e., 180o).